COVID-19 Updates
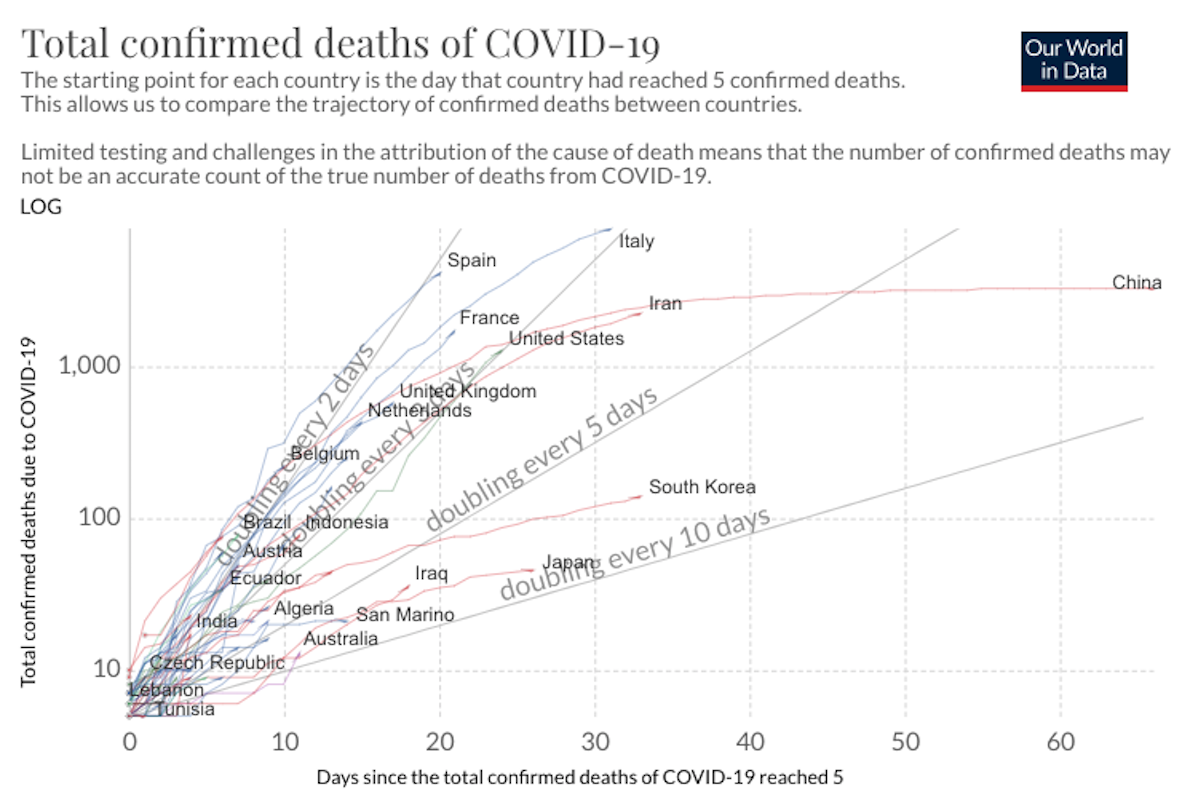
COVID-19 Science Update for March 27th: Super-Spreaders and the Need for New Prediction Models
Absent isolation or other precautionary measures, the average socially active COVID-19 infectee will transmit the disease to an average of about 2.4 people. i.e., the R0 value is 2.4. But super-spreaders can spread a disease to dozens or hundreds.
· 11 min read
Keep reading
Musk Is Enticed by the Lunar Siren
Robert Zubrin
· 18 min read
Collision with Reality
Phillip W. Magness
· 10 min read
Melania at the Mic
Brian Stewart
· 5 min read
Erasing the Word “Woman”
Karleen Gribble
· 24 min read
Arrest and Moral Pageantry
Julia Friedman
· 8 min read
A Justified Assassination
Iona Italia
· 6 min read