Epidemiology
A Baseless Attack on Groundbreaking RSV Therapies
A member of the CDC’s Advisory Committee on Immunization Practices is spreading misinformation about new antibody treatments that protect children from serious Respiratory Syncytial Virus infections.
Most parents reading this have likely heard of RSV—or Respiratory Syncytial Virus—which causes infections of the lungs and respiratory tract. It is estimated that RSV leads to more than 100,000 deaths around the world every year.
Unlike SARS-CoV-2, the virus that causes COVID-19, RSV isn’t a novel pathogen; it’s been infecting people since the 1950s (after it had first been identified in chimpanzees). Nor is it nearly as deadly as COVID-19. But it does have one worrying quality that should be forcing public-health professionals to take it seriously: The most severe RSV infections among otherwise healthy patients typically afflict very young children—including babies and toddlers. In the United States, tens of thousands of children with RSV end up in hospitals annually.
The 2024–25 RSV season should have been marked as a triumph of evidence-based public health: For the first time, we had effective preventative therapies to protect infants—specifically, (1) the maternal RSV vaccine (produced by Pfizer under the trade name Abrysvo), and (2) anti-RSV monoclonal antibodies that are being administered to infants. In fact, there are now two types of anti-RSV monoclonal antibodies available, clesrovimab and nirsevimab; marketed as Enflonsia by Merck and Beyfortus by Sanofi, respectively.
In one recently published study—comparing infants aged 0–7 months during 2024–25 with pre-COVID-19 cohorts—RSV-associated hospitalisations were found to have dropped by roughly thirty to forty percent, with even steeper declines of about fifty percent in newborns under two months old. Similar results have been observed in other jurisdictions. According to Canadian media reports, for instance, data collected in Quebec indicate that nirsevimab was “more than eighty per cent effective” at preventing emergency room visits and hospitalisations.

(A note on terminology: anti-RSV monoclonal antibodies are not vaccines, but rather human immune-system proteins that help ward off infection in the short term. Unlike vaccines, they do not help program a patient’s body for long-term immune responses. But from a patient’s perspective, they may be understood as being similar to vaccines.)
Instead of building on this success, however, a member of the American scientific advisory system tasked with guiding federal public-health policy has chosen to conjure a spurious controversy. In a recent analysis, Dr Robert Malone, a sitting member of the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP)—along with Dr Yaffa Shir-Raz, an Israeli researcher and health journalist—recently claimed that the CDC had manipulated data, hid safety signals, and misled ACIP about the risks of anti-RSV monoclonal antibodies. These charges deserve scrutiny because misinformation originating from within the US federal advisory system poses a uniquely corrosive threat to evidence-based policy.
On 20 August, Dr Shir-Raz, a vaccine sceptic, published an essay on the web site of the Brownstone Institute, a think tank with a history of promoting dubious anti-vaccine misinformation. In it, she described a June 2025 meeting of ACIP, the first held under the auspices of newly appointed Health Secretary Robert F. Kennedy, Jr.
“One of the most significant items on the agenda was whether to recommend Merck’s new RSV monoclonal antibody, clesrovimab, for routine use in healthy newborns,” she wrote. According to Dr Shir-Raz, the CDC presentation that day failed to mention what she describes as “a consistent and notable imbalance in deaths [of infants] between treatment and control [groups]” in clinical trials.
Dr Malone, who has his own history of spreading dubious information about vaccines, then wrote on Substack that he regretted having voted in favour of clesrovimab usage at that meeting; as he now believed that ACIP’s favourable decision was based on “manipulated data.” The alleged manipulation was so egregious, he claimed, that, in his words, “I will no longer be able to trust that what is presented in CDC summaries to the ACIP is transparent, accurate, and unbiased.”
US RSV immunizations + monoclonal antibodies cut infant hospitalizations ~30–40% last season.
— Jake Scott, MD (@jakescottMD) August 21, 2025
Yet ACIP member, Dr. Malone, and Dr. Shir-Raz are undermining this progress with manipulated stats and false 'hidden death' claims. Read the primary sources and it all falls apart. 🧵
The centrepiece of Dr Malone and Dr Shir-Raz’s shared analysis concerns seizures in infants who received nirsevimab. They believe the CDC improperly split safety data into two age groups in order to hide a concerning trend that emerges when the groups are combined.
The CDC’s Vaccine Safety Datalink team monitored 36,719 infants who received nirsevimab, and found elevated but statistically insignificant seizure rates in both neonates (infants younger than four weeks old) and older infants. Among the neonates, there were four seizures, as compared to the two that would have been expected based on the incidence in the population at large. Among the older infants, aged between 38 days and eight months, there were five seizures—again, as compared to the two that would have been expected. Neither result was deemed to be statistically significant under generally employed analytical standards.
Dr Malone and Dr Shir-Raz argue that by combining these two groups and then performing the corresponding statistical analysis using the pooled data, a “statistically significant” result is thereby achieved, one that demonstrates a “nearly a four-fold higher risk of seizures shortly after the injection.” They present this as evidence of CDC manipulation, as the pooled data analysis “was never presented to the committee.”
But performing the kind of pooled analysis they focus on would be epidemiologically inappropriate, and so the result they derive with their own method is meaningless.
Moreover, even the (inappropriately construed) risk analysis they present rests on the extreme rarity of the negative events in question: just nine seizures versus four across tens of thousands of infants. In such a statistical context, a single data point can flip the results dramatically. This is why the confidence interval associated with their pooled analysis is so broad—spanning a risk-multiplier range from barely above one to nearly thirteen—i.e., anywhere from trivial to catastrophic.
But the most important point here is that the CDC stratified its analysis for sound scientific reasons. Older infants in the United States routinely receive multiple vaccines on the same day as they (now) get nirsevimab. More than eighty percent get same-day immunisations including DTaP, Hib, pneumococcal, polio, and rotavirus vaccines. But only about twenty percent of neonates receive vaccines simultaneously. Since many vaccines are well-established fever triggers, and febrile seizures are documented vaccine side effects, pooling these two populations would serve to misattribute vaccine-induced seizures related to vaccines to the monoclonal antibody.
In other words, the CDC stratified its analysis to prevent confounding bias. What Dr Malone and Dr Shir-Raz call manipulation is actually standard epidemiological practice. Anyone familiar with paediatric public health would know this—including (perhaps, especially) scholars such as this pair, who present themselves as knowledgeable of the potential health risks associated with vaccines.
The two authors further confuse matters by claiming that Merck’s clesrovimab “is nearly identical in structure and function” to nirsevimab. This assertion, which forms their basis for extrapolating safety concerns between the two products, is simply wrong.
While both products are long-acting monoclonal antibodies targeting the RSV fusion protein, they bind to different sites on the targeted molecule (thereby producing different resistance profiles and potentially different safety characteristics). The two drugs are also dosed differently, are produced using different processes, and were approved through separate clinical development programs (which is entirely appropriate since they represent different approaches to RSV prevention). The claim that the two are “nearly identical” reveals a fundamental misunderstanding of monoclonal antibody development.
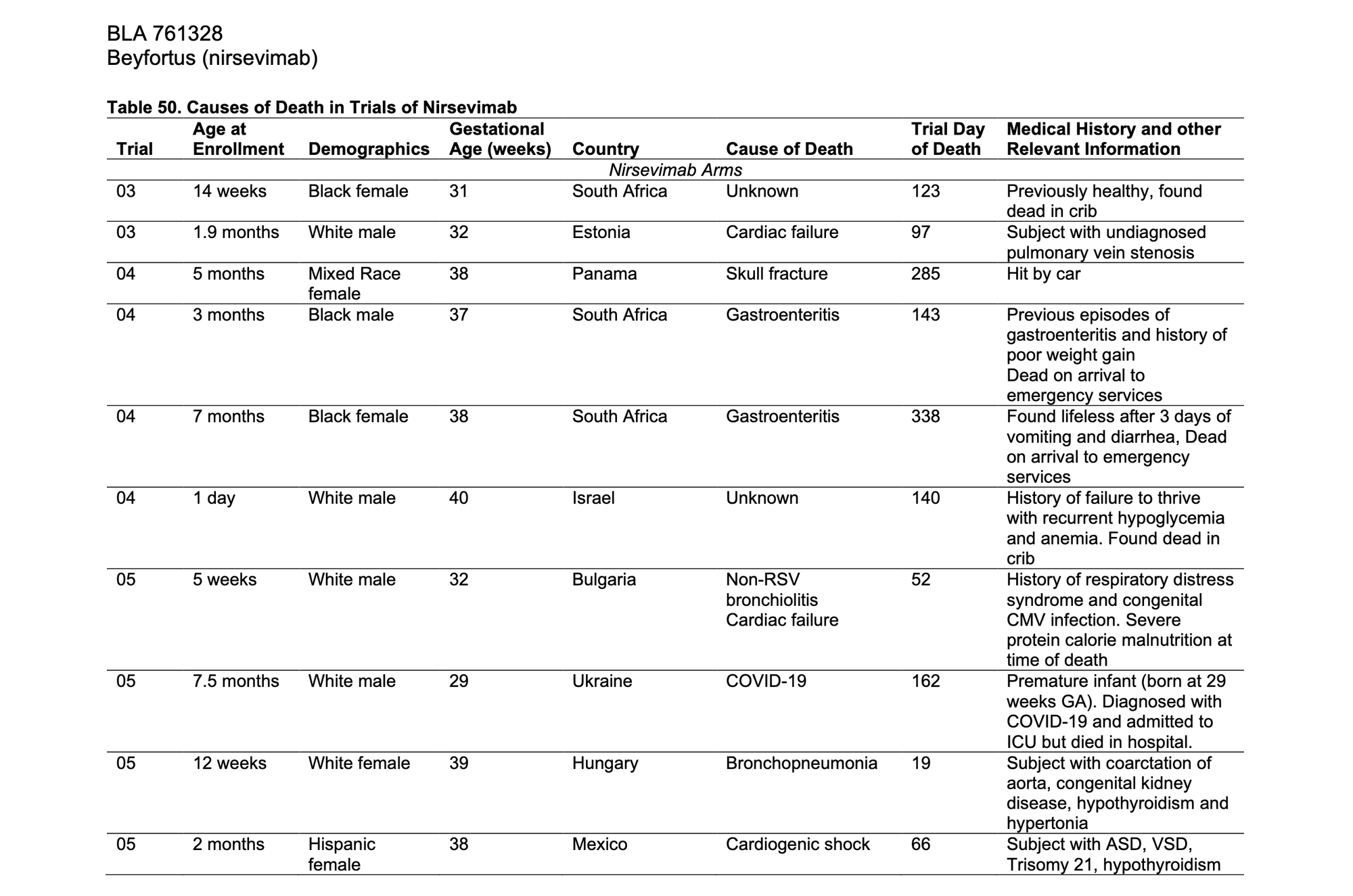
The most inflammatory aspect of the critics’ analysis concerns the deaths documented in clinical trials. In Dr Shir-Raz’s article, “What the ACIP Wasn’t Shown”—subsequently reprinted and endorsed on Dr Malone’s Malone News Substack—there appears a section luridly subtitled, “Deaths Hidden in Footnotes.” This contains the claim that data concerning child deaths were “hidden” in obscure portions of a 2023 supplementary appendix produced by nirsevimab researchers whose work appeared in the New England Journal of Medicine (NEJM). Dr Shir-Raz writes of the associated clinical trials: “In total [there were] twelve deaths among 3,710 nirsevimab recipients versus four deaths among 1,797 controls—a mortality rate of 0.32% in the treatment arms compared to 0.22% in the control arms. The imbalance may appear small in absolute terms, but it was unexpected, and it runs consistently in one direction.”
What is omitted is that every one of these deaths was individually reviewed by FDA regulators, and none were attributed to nirsevimab. (One of the deaths was due to a car crash, for instance.) These conclusions are worth quoting at some length, as they attest to the rigorous nature of the processes that Dr Shir-Raz and Dr Malone seek to impugn:
The causes of death were varied and were not related to the same System Organ Class [i.e., broad medical category]. In the nirsevimab group, the causes of death appeared to be related to a cause other than the drug product for eight subjects: three subjects with congenital cardiac disease, two with untreated gastroenteritis, one with COVID-19, one with a tumor, and one with a skull fracture. Another two subjects died of lower respiratory tract infections. Of these two subjects, one had underlying severe protein calorie malnutrition and the other had multiple underlying conditions. One subject died at home and the other had been removed from the hospital against medical advice. Both deaths were likely related to underlying conditions and not to nirsevimab. Finally, two subjects in the nirsevimab arm died of ‘unknown’ causes after being found dead in their crib. One of these subjects had multiple prior hospitalizations, and his health care provider theorized that this subject had an underlying congenital metabolic or chromosomal anomaly. The other subject was healthy without previous issues. The death was on Day 123 and was not temporally related to receipt of nirsevimab. The circumstances of death are consistent with sudden infant death syndrome; however, the cause is unknown, and the autopsy result was not available for review. The causes of death in the nirsevimab are consistent with the causes of death in the control arms. In the opinion of the reviewer, none of the deaths appeared related to the trial drug.
The sensational-seeming suggestion by Dr Malone and Dr Shir-Raz that deaths have been “hidden” is without basis. As the text above indicates, drug regulators are transparent about the follow-up and background information they access in regard to patients enrolled in clinical trials.
In “What the ACIP Wasn’t Shown,” the two authors raise suspicions about “an additional [child] death on day 487, after the infant had formally discontinued participation [in the clinical trial] at the physician’s instruction.” But in fact, such information was properly documented in both NEJM supplementary materials and Australian regulatory documents as an out-of-window event. The Australian review noted that “an additional [nirsevimab-recipient] death occurred due to a road-traffic accident 440 days after the clinical trial began,” clearly outside the 360-day primary-safety-analysis period.
This is transparent reporting, not concealment. And the ACIP’s focus on post-marketing surveillance (i.e. the ongoing process of monitoring a drug after it has been approved and released to the public), as opposed to re-reviewing every clinical-trial death, was entirely appropriate for a committee charged with evaluating incoming safety data. As an ACIP committee member, Dr Malone presumably knows this.
While Dr Malone and Dr Shir-Raz seek to generate controversy from statistical noise, real-world evidence demonstrates there are clear population-level benefits associated with anti-RSV therapies. More than 74,000 infants have been monitored in post-marketing surveillance with no confirmed safety signals detected. The estimated thirty to forty percent reduction in RSV hospitalisations represents thousands of prevented admissions, shortened hospital stays, and families spared the trauma of watching their infants struggle to breathe.
It is hardly unusual that a small share of doctors in the United States (or any jurisdiction) would hold fringe views about this kind of safe and effective medical therapy—nor that they would give voice to such misguided concerns in the lay media. What is unusual in this case is that the misinformation is being promoted from within the advisory system itself, thanks to Dr Malone’s ACIP affiliation. When ACIP members misrepresent basic pharmacology, ignore epidemiological principles, and misunderstand surveillance methodology, they undermine the expertise the committee system is designed to provide.
Good public health policy requires adherence to the totality of evidence: biologically sound study design, transparent case-level review, and real-world population impact. The RSV immunisation program delivers on all three criteria. Rolling back RSV availability based on baseless concerns would do harm to American children. Perhaps more importantly, it would set a dangerous precedent in regard to how America’s scientific advisory-committee system might be undermined—or even co-opted—by peddlers of junk science.






