Environment
Should We Be Worried About GMOs?
GMOs interact with wider global systems that they could potentially undermine, with terrible consequence.

After shaping life on earth for billions of years, evolution via natural selection is in decline and being replaced by intelligent design. For the last 12,000 years, the survival of species has been primarily determined by their usefulness, and vulnerability, to human beings. Now, finally, we have found a way to do away with even this vestige of our biological past and to design species from the top down, working out what traits are desirable and undesirable to us and genetically engineering organisms accordingly. How much should this worry us?
For many, the answer is “a lot.” For instance, Nassim Taleb has made dire warnings about the application of GMOs, arguing that they “represent a public risk of global harm” given their potential to produce unrecoverable losses or “ruin.” According to him this justifies a highly precautionary response of “avoid at all costs,” unless and until GMOs have been proven to be safe beyond doubt.
GMOs pose such risks, and justify such a response, because, Taleb argues, their impact cannot be localized or contained. GMOs interact with wider global systems that they could potentially undermine, with terrible consequences. In particular, “the cross-breeding of wild-type plants with genetically modified ones prevents their disentangling, leading to irreversible system-wide effects with unknown downsides,” while “healthwise, the modification of crops impacts everyone.”

Against such claims, supporters of GMO development point out that great care is taken to avoid the escape of GMOs, that the hypothetical risks cited against them seldom have anything like a scientific foundation and that GMOs have many benefits, including their potential to support global environmental and health systems by reducing the demand for agricultural land, the use of pesticides and the prevalence of malnutrition and disease. However, none of these arguments implies that GMOs couldn’t have the kind of systemic impacts that Taleb and others have worried about, so, how can we be so sure that they are safe?
Sometimes, the answer is straightforward. All one has to do is remember that the only reason most traits are selected for in a GMO is that they are in the interest of human beings, not that they benefit the GMO itself.
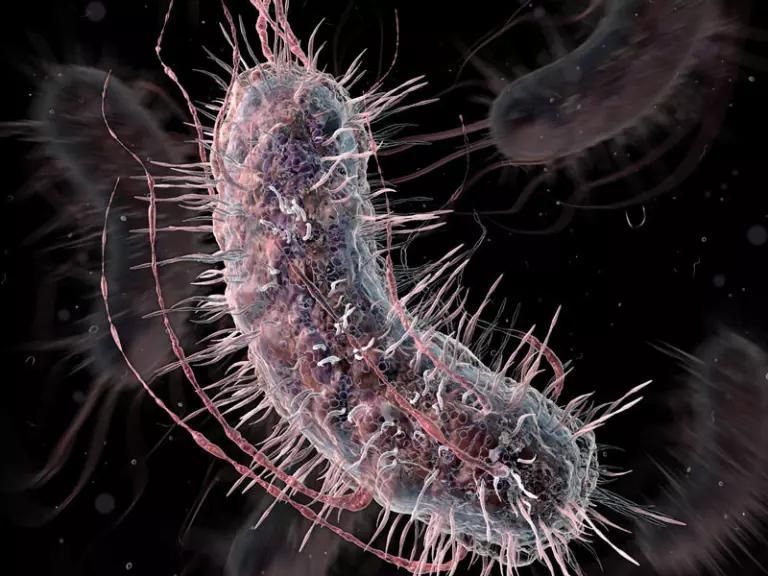
Consider Escherichia coli, which has been genetically modified for the production of a variety of proteins, including insulin and chymosin (the enzyme responsible for making many kinds of cheese). The ability to produce these proteins is important to humans, and a lot of research has been done to understand the conditions that help produce as much as possible.
However, for the bacteria, the production of these proteins is of no value whatsoever and places a metabolic burden on the cells that substantially decrease their rate of division. Therefore, outside of carefully optimized laboratory conditions, such bacteria will be significantly disadvantaged and unable to compete against their wild cousins. Similarly, many crops have been modified to make their fruits bigger, sweeter, or more nutritious, but usually in ways that reduce the crops’ ability to reproduce naturally, or that even render it sterile.
Hence, despite the fact that genetically modified bacteria are technically compatible with wild-type organisms, the genes that had been inserted into such GMOs would be strongly disfavored by natural selection, and hence disappear from any hybrid population, posing no risk to wild populations or their ecosystems.
Of more concern are organisms whose traits confer adaptive advantages, such as disease or pest resistance. These GMOs have the potential to provide substantial benefits, such as aiding in the preservation of biodiversity, eradicating agricultural diseases, and even contributing to the rescue of keystone species from extinction. However, the problems that these organisms seek to resolve are themselves pervasive, and thus require solutions with an equal potential for widespread, systemic impact. Unfortunately, it is this very quality that could result in a GMO posing a systemic threat. By outperforming non-GMOs, these organisms could overtake or substantially corrupt wild populations. Additionally, the containment of the organism itself may be insufficient to prevent systemic consequences, as the presence of the organism may alter the nature of the threats present in the ecosystem.

For instance, consider Q124, a variety of sugarcane cultivated in Central Queensland with resistance to the fungal disease orange rust. Q124 was initially effective at increasing crop yields and became the dominant agricultural monoculture as a result. However, in the year 2000, an outbreak of an evolved strain of the disease overcame the resistance of Q124 and resulted in a dramatic reduction in the size of the sugarcane harvest that year across Central Queensland and the costliest disaster to hit the industry.
The initial adaptive advantages of Q124 led to a decrease in genetic diversity in the area, as it outperformed other varieties of sugarcane, resulting in 85 percent of the cultivated population in Central Queensland being Q124 and, therefore, vulnerable to the evolved disease. This example demonstrates the potential for adaptive traits to both provide short-term advantages that lead to a widespread monoculture but then ultimately exacerbate long-term threats, with the potential for ruinous systemic impact.
This seems to provide an excellent example of the kind of systemic risk that worries Taleb and others. There is only one problem – Q124 is not a genetically modified organism. Developed as part of the Queensland Bureau of Sugar Experimentation Station’s sugarcane breeding program, its parent varieties were selected to optimize disease resistance and yield, but at no point was genetic engineering involved in conferring these adaptive traits. Taking a precautionary approach to the regulation of GMOs would, therefore, do nothing to prevent bad outcomes such as this.
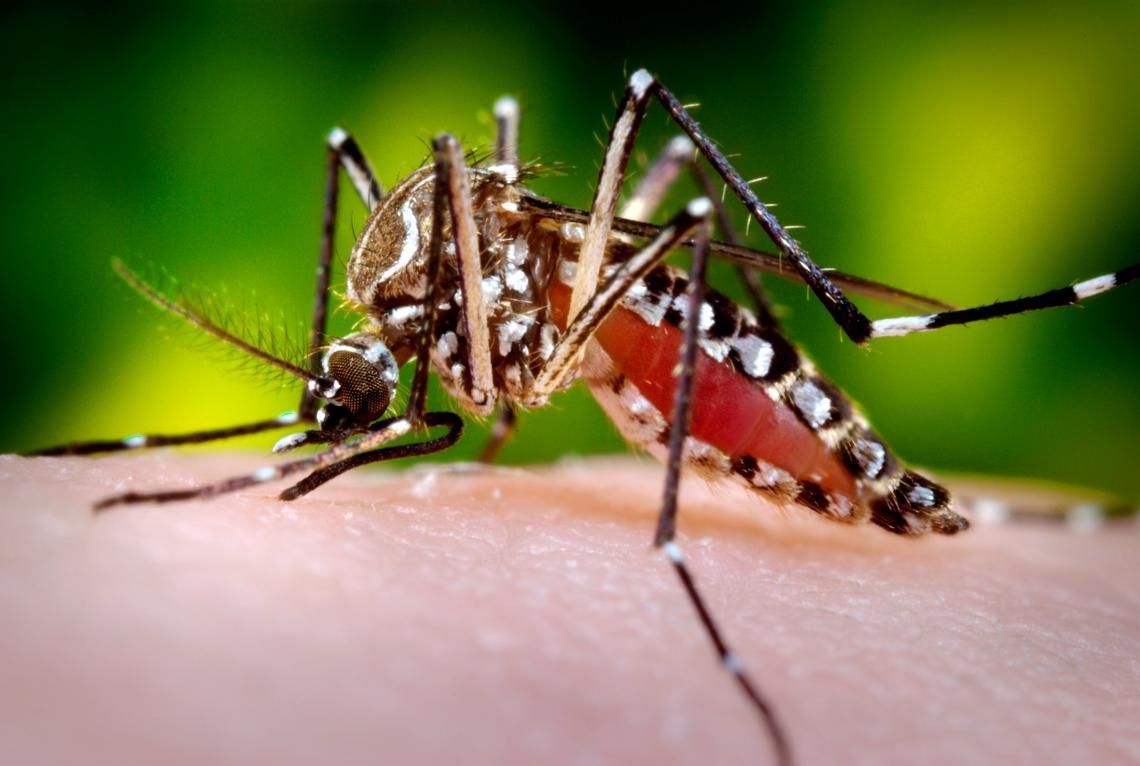
Consider another example (not a trick one this time). The bioscience company Oxitec has been developing GM mosquitoes that have the potential to eliminate populations of the Aegypti mosquito that spreads many tropical diseases. These mosquitos, however, have proven highly controversial, with many advocating a precautionary approach to their release. But such concerns almost universally relate to the potential that GM mosquito genes might escape into the wild or even into humans. Such concerns miss the critical fact that the trait these GM mosquitos possess is one that will almost certainly kill them before reaching adulthood, something that is clearly highly disadvantageous to the mosquito and therefore one that will not survive without continuous human support.
Nevertheless, the release of these GM mosquitos will undoubtedly have at least one significant systemic impact; it will remove a species from an ecosystem. Right now, we have little knowledge about what implications that might have, but, strange as it sounds, it is not impossible that mosquitos play an important ecological role. However, such impacts aren’t really anything to do with the use of GMOs per se – humans have been trying to wipe out mosquitos for a very long time. The only thing that the use of GMOs changes is that we are now more likely to succeed.
Taking a precautionary approach to GMOs, therefore, risks going too far while simultaneously not going far enough. Not only does it overlook the potentially important benefits that GMOs can bring and the many ways that the risks they pose can be mitigated, but it also fails to acknowledge the real source of the systemic risks that we should be most concerned to avoid. A regulatory regime that emphasized the potential role that a GMO, or any novel organism, could come to play in wider systems, and reviewed the practices that would be involved in its cultivation, would be far more appropriate. This would allow for the development of safe and beneficial technologies while remaining alive to the risks they pose.